Abstract
Background: Chimeric antigen receptor T-cells (CAR-T) have emerged as a promising treatment for children with relapsed/refractory acute lymphoblastic leukemia (ALL). While CAR-T outcomes have been published, little data exist on how to manage these patients for the weeks to months between T-cell collection and CAR-T infusion. Unlike stem cell transplant (SCT), where higher burden of disease is associated with poor outcomes and is often a contraindication, successful outcomes for children with high disease burden pre-CAR-T have been demonstrated. Thus, traditional high-intensity chemotherapy for relapsed ALL that aims to minimize disease burden but carries significant morbidity may not be the best option for pre-CAR-T populations. We thus compared our population-based experience in using high vs. low-intensity chemotherapy regimens to bridge patients in terms of toxicity, inpatient days, and success in reaching CAR-T infusion.
Methods: The Hospital for Sick Children (Sickkids) is the provincial referral centre for cellular therapy for children in Ontario, Canada. All Ontario children referred to Sickkids between 2014-2018 for first T-cell collection with intent to proceed to CAR-T therapy were included and followed until CAR-T infusion, decision to pursue alternative therapy, or death. Bridging regimen details were collected and classified as low vs. high intensity based on whether such therapy was likely associated with >7 days of neutropenia. Disease and outcome variables were compared between high vs. low intensity regimens using Chi squared, Fisher's exact, or Wilcoxon tests.
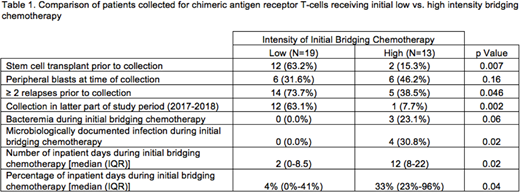
Results: The cohort included 32 patients with a median age of 9.7 years at the time of first T-cell collection [interquartile range (IQR) 6.0-12.3]. The median number of previous relapses was 2 (IQR 1-2), 14 (44.0%) had undergone prior SCT, and 4 (12.5%) had Down syndrome. The vast majority of patients (28, 87.5%) were successfully bridged to receive CAR-T therapy with a median time to infusion of 81 days (IQR 60-105). Two patients experienced manufacturing failure and pursued SCT instead, 1 died of toxicity, and 1 was still awaiting infusion at the end of the study period. Low-intensity bridging regimens were used following collection for 19 (59.4%) patients, most often based on low-dose intravenous methotrexate, 3-drug induction (vincristine/steroids/asparaginase), or maintenance therapy. The most common high-intensity regimens included cyclophosphamide/etoposide, high dose cytarabine, or 4-drug induction (vincristine/steroids/asparaginase/anthracycline). Patients receiving high-intensity therapy did not seem to have more aggressive disease prior to starting bridging treatment (as indicated by peripheral blasts, number of previous relapses, prior SCT) that would have justified choosing high-intensity treatments. Patients receiving initial high-intensity regimens were however more likely to have been collected in first half of the study period (Table 1). Patients receiving initial high-intensity regimens also developed more microbiologically documented infections and experienced a greater number of inpatient days (Table 1). Excluding patients experiencing manufacturing failure or still awaiting CAR-T at the end of the study period, the likelihood of receiving CAR-T also did not vary [high-intensity regimen - 11/12 (91.7%) vs. low-intensity regimen - 17/17 (100%); p=0.41].
Conclusions: We demonstrate in our population-based cohort of heavily pre-treated and high-risk patients that initial low-intensity chemotherapy had a very high likelihood of successfully bridging children to CAR-T infusion. Low-intensity bridging regimens were associated with lower rates of toxicity and higher quality of life as indicated by fewer inpatient days. Low-intensity regimens should be considered the first line option in this population.
Grupp:Jazz Pharmaceuticals: Consultancy; Adaptimmune: Consultancy; University of Pennsylvania: Patents & Royalties; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding. Maude:Novartis Pharmaceuticals Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal